TIDE clinical trial opens in France
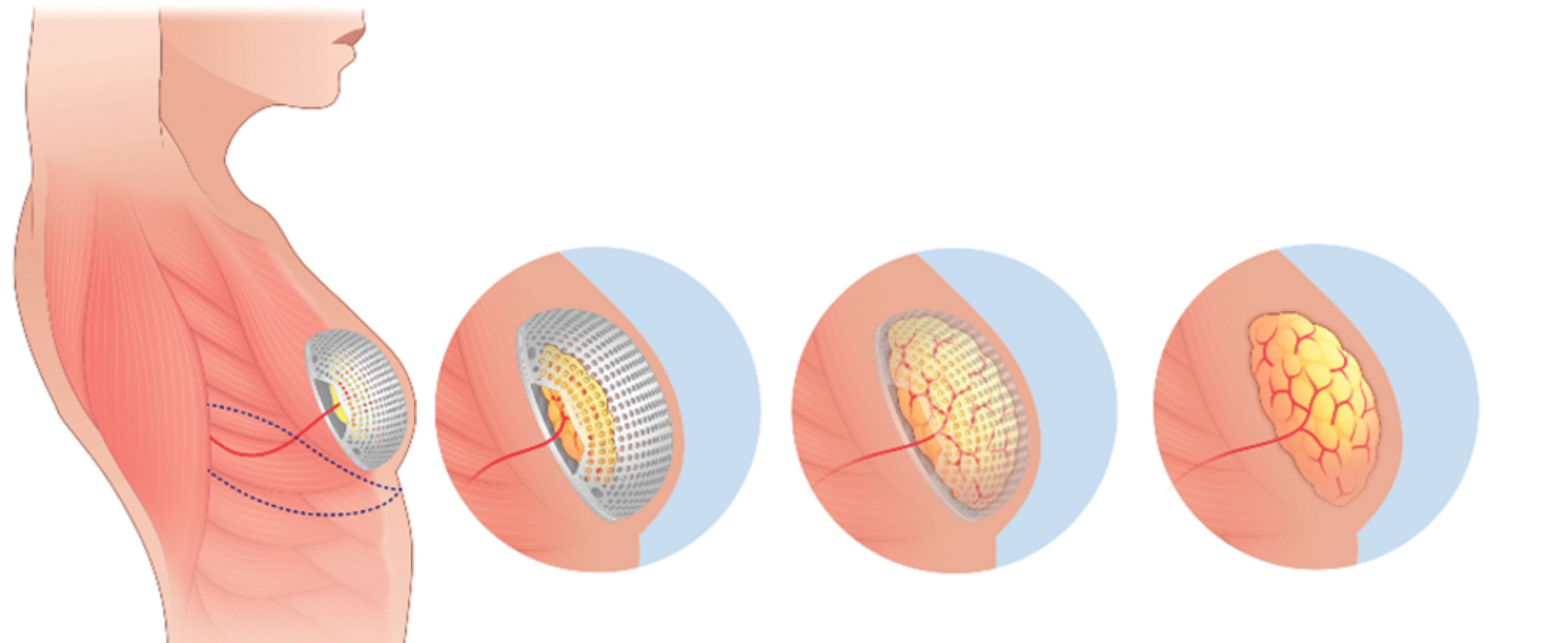

LATTICE MEDICAL announces the opening of the TIDE clinical trial in France for the MATTISSE device, a natural, resorbable prosthesis for patients requiring breast reconstruction after total mastectomy.
Lille, 19 September 2023 – LATTICE MEDICAL announces the opening of its TIDE clinical investigation in FRANCE. The trial concerns its innovative MATTISSE device, a resorbable bioprosthesis enabling the regeneration of autologous adipose tissue in patients requiring breast reconstruction after total mastectomy as part of their breast cancer treatment. Once implanted, MATTISSE regenerates a vascularised adipose flap within 6 months, harvested close to the breast area. This is a simplified surgical procedure compared with techniques using flaps, which require an hour and a half to perform. It is also less invasive and leaves fewer after-effects because it uses the same scar as a mastectomy. It also leaves few traces on the donor site. Thanks to regeneration, the technique requires very little fatty tissue initially, and the resorption of the implant makes reconstruction more natural, with the gradual disappearance of the device giving way to autologous regenerated tissue.
After Georgia in 2022, France is the second country to authorise our First-In-Human (FIH) interventional study for our MATTISSE implant. The TIDE study aims to collect and study all the data relating to the safety of the MATTISSE implantable medical device, as well as performance-related data such as fat tissue regeneration and patient quality of life.
The clinical trial of this innovative medical device is now open in France and eligible for women requiring breast reconstruction after total mastectomy. All of the study’s eligibility criteria will be verified during an initial consultation with the investigating centres in Lille, Paris and Strasbourg.
LATTICE MEDICAL is an implantable medical device company which develops and manufactures a breakthrough technology in the field of autologous adipose tissue reconstruction.
Breast cancer affects 1 in 8 women today. In 40% of cases, the treatment is surgical with a complete or partial mastectomy, and only 20% of women will benefit from reconstruction, because these operations have drawbacks and are costly for healthcare systems. That’s why LATTICE MEDICAL has developed the MATTISSE medical device, which allows tissue regeneration and resorbs in the body after breast reconstruction, thus avoiding additional surgery and long-term risks for patients.
To download the PR, visit 👉🏼: PRESS RELEASE TIDE STUDY – LATTICE MEDICAL
If you would like more information, please contact us at clinic@lattice-medical.com!

