TIDE clinical study

The TIDE study is a clinical trial evaluating the efficacy and safety of MATTISSE.
This is the first time MATTISSE is implanted on a human being. MATTISSE is a resorbable tissue engineering chamber (prosthesis) designed to help regenerate your own tissue. During reconstruction, your fat tissue will be harvested from the intercostal region and implanted into the tissue engineering chamber. Over time, your own tissues will regenerate inside the chamber and grow to restore volume to your breast.Once your tissues have developed, the chamber will reabsorb over the months.
Patients requiring further information on their eligibility are invited to contact the Lille University Hospital directly: flavien.gautron@chu-lille.fr or gaelle.guyot@chu-lille.fr and complete the following questionnaire: https://yl3m9s1wlgs.typeform.com/to/E6kXBUkr
The study in a few figures
research centres
different countries
months of study
patients included at term
The study process
To be considered valid, a scientific study has to follow a strict process.
Inclusion visit
Medical examination with your surgeon. He or she will tell you about the study and make sure you can take part. If you agree, you can sign a consent form.
Surgical procedure
Breast reconstruction after planning with your doctor.
Exit and follow-up consultations
Leaving hospital, consultations every 15 days until healing. Then visits at 3 months, 6 months, 1 year, 2 years and 3 years after surgery. The surgeon will check that everything is going well with a clinical examination. You will be asked to complete questionnaires. An MRI scan will be performed at each visit.
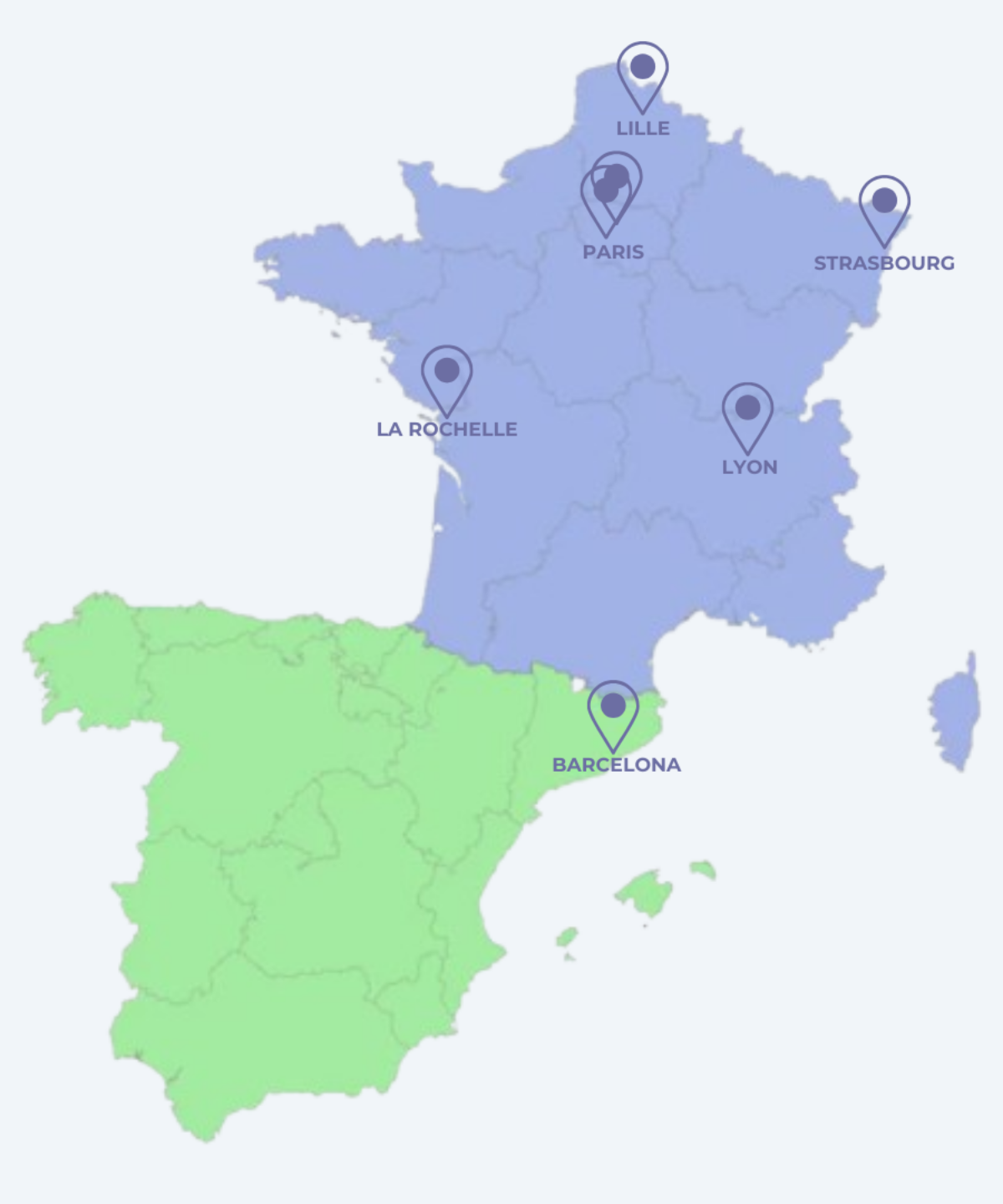
Centres taking part in the study
- Center Hospitalier Universitaire de Lille - Plastic and reconstructive surgery department 2 Av. Oscar Lambret, 59000 Lille 03 20 44 59 62
- Hopitaux Universitaires de Strasbourg - Breast reconstruction department Hôpital de Hautepierre, 1 Av. Molière, 62000 Strasbourg Cedex 03.88.11.67.68
- Hôpital Tenon AP-HP - Plastic, reconstructive and aesthetic surgery unit 4 rue de la Chine, 75020 Paris 01 56 01 70 00
- Gustave Roussy - Plastic Oncology and Reconstructive Surgery Department 114 Rue Edouard Vaillant, 94800 Villejuif 01 42 11 65 36
- La Clinique de l'Atlantique - Ramsay Santé 26 Rue Moulin des Justices, 17138 Puilboreau 05 36 28 85 00
- Hospital de la Santa Creu i Sant Pau Barcelona Carrer de Sant Quintí, 89, Horta-Guinardó, 08025 Barcelona, Spain 93 556 57 75
- Léon Bérard center 28 Rue Laënnec, 69008 Lyon 0478782828
Would you like to receive
our newsletter?
Receive our newsletter directly in your mailbox

